In line with the FTC, Neora has operated as a pyramid scheme since inception as Nerium Worldwide in 2011.
Up till earlier this 12 months Neora glided by Nerium Worldwide. BehindMLM reviewed Nerium Worldwide again in 2014.
In our assessment we famous a number of autoship recruitment considerations;
Talking of autoship, it conveniently brings us to the most important red-flag I see in Nerium’s compensation plan.
For causes not totally clear to me, Nerium require associates to buy a month-to-month 80 PV minimal autoship order or generate 200 PV in retail buyer orders to qualify for commissions.
Shouldn’t this be the opposite means round?
The Quick Begin Bonus for instance, which is principally “join autoship and recruit 3 associates who do the identical”, completely illustrates potential issues.
We revisited Neora as Nerium and printed an up to date assessment in February.
To our dismay, within the 5 years since our authentic Nerium assessment, Neora failed to handle its give attention to affiliate autoship recruitment.
Following their very own investigation, the FTC concluded;
Since its inception, Nerium has operated as an unlawful pyramid scheme.
In contrast to a reputable multi-level advertising enterprise, Nerium’s compensation scheme emphasizes recruiting new BPs over the sale of merchandise to shoppers exterior of the group.
Nerium’s enterprise mannequin makes it unlikely that BPs can earn cash by promoting product to exterior shoppers in response to real demand.
“Outdoors shoppers” being retail prospects, that are usually ignored in an affiliate autoship recruitment mannequin.
With respect to retail gross sales, evaluation of fee quantities reveals why Neora distributors (known as BPs) would go for autoship recruitment over retail.
BPs who don’t join auto-delivery obtain no low cost on their product purchases, that means that gross sales from their private stock wouldn’t be worthwhile even at full retail worth.
Even for BPs who join auto-delivery, the revenue margin is often slim or non-existent.
All in all, Neora had admitted to the FTC that
lower than 1% of all rewards paid by the corporate include commissions paid on the sale of merchandise to Retail Clients.
As is true in all pyramid schemes, nearly all of Neora distributors made no cash.
Listed below are the information and figures quoted by the FTC pertaining to Neora distributor losses;
In line with Nerium’s most up-to-date reporting, lower than 5% of BPs in the USA earn extra from Nerium than they pay in charges and product purchases.
At the very least 92% of Nerium’s BPs have give up, with half leaving the corporate inside six months or much less.
The difficult actuality of retailing and recruiting has meant that only a few Nerium individuals get above even the primary rung on the organizational ladder.
Though there at the moment are eighteen ranges within the Nerium enterprise alternative hierarchy, Nerium studies that greater than 85% of BPs have by no means reached the second rank.
In the meantime, the overwhelming share of rewards accrues to the few people who attain the highest ranks of Nerium’s group.
And what little cash Nerium distributors do make, are sometimes clawed again in charges.
Nerium costs its BPs varied charges which usually are far larger than any compensation they pay the BPs.
Particularly, Nerium BPs must pay out of their very own pockets charges for gross sales aids, enterprise playing cards, letterhead, and registration at a number of conferences, together with Nerium’s annual multi-day “GetReal” convention.
Attributable to these quite a few charges, in keeping with Nerium’s most up-to-date knowledge, greater than 95% of Nerium BPs paid extra to Nerium every month than they earned in commissions and bonuses.
The FTC has additionally gone after Neora for misleading advertising practices.
Nerium promotes its enterprise by misrepresenting that BPs can earn a considerable earnings and obtain monetary independence.
Regardless of guarantees of economic independence and six-figure incomes, lower than 1% of “lively” Nerium BPs averaged earnings of no less than $530 in any given month.
A much smaller quantity earn that quantity each month, which might nonetheless solely end in an annual gross earnings of lower than $6,400.
For sure no one is reaching monetary independence on $6400 yearly or much less.

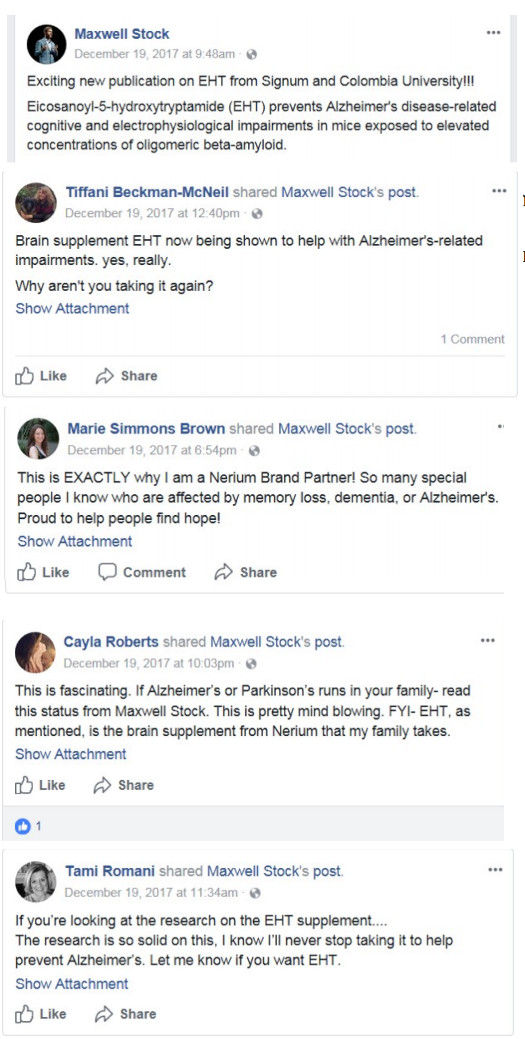
Signum, a service provider producer, equipped Neora with their ME Sports activities and Nerium EHT merchandise.
Signum launched EHT in 2014
with enter from Nerium’s advertising employees, conveying that EHT may assist with CTE, Alzheimer’s illness, and Parkinson’s illness.
Defendants even have claimed that EHT is scientifically confirmed to supply customers vital well being advantages.
To this point nevertheless, the one research relating to EHT have concerned rodents.
Defendants relied solely on rodent research, however they repeatedly conveyed to Nerium BPs who purchased and offered Nerium EHT that these rodent research have been enough to determine the efficacy of EHT on people.
Moreover, Defendants have falsely implied that Princeton College was concerned within the improvement of EHT.
Defendants even have claimed that Signum has succeeded in creating medical breakthroughs regarding Alzheimer’s and Parkinson’s the place precise pharmaceutical producers have failed.
Signum initially approached pharmaceutical corporations to distribute EHT.
The pharmaceutical corporations approached insisted human trials be carried out, with the intention to validate EHT associated advertising claims.
Relatively than conduct these trials, Signum dumped EHT onto Nerium distributors.
Signum initially deliberate to proceed with scientific testing however finally rejected that method as too pricey.
As an alternative, Signum deliberate for Nerium’s community of a whole bunch of 1000’s of BPs—who have been incentivized to recruit others to the scheme and customarily had no scientific background—to distribute EHT Merchandise and unfold claims about it by “phrase of mouth.”
Neora’s (then Nerium) and Signum’s partnership started in 2014. For essentially the most half, Neora dealt with Signum’s internet presence, model consciousness and promoting campaigns.
Defendants formally introduced their partnership and the launch of Nerium EHT at Nerium’s GetReal Convention for BPs in April 2015.
Presenting EHT to 1000’s of BPs on the April 2015 GetReal Convention, a Signum consultant touted the numerous funding Signum acquired from the Michael J. Fox Basis and linked the declare that EHT protects and stabilizes proteins within the mind to the mind injury suffered by NFL participant Junior Seau.
Instantly afterwards, Jeff Olson informed the group, “lots of issues you may’t say, we’ll speak about that afterward, as a result of all these issues you may’t say – it does!”
This pseudo-compliance result in Nerium itself not offering data on Nerium EHT on their web site.
In an April 2015 e mail, Nerium’s main BP requested Defendant Jeff Olson and Nerium’s advertising director whether or not content material from the ME Sports activities web site could be copied into Nerium’s Digital Library, permitting BPs to simply present that data to prospects.
Nerium’s advertising director responded that a number of the content material is barely on Signum’s web site “on function” as a result of Nerium “can not use/say [it] from reg[ulatory] standpoint.”
Defendants understood that even when Nerium presupposed to forbid sure illness claims, the Nerium BPs would nonetheless unfold these claims in speaking to potential prospects and recruits.
In the long run Nerium’s pseudo-compliance was for naught.
“The Michael J Fox Basis for Parkinson’s analysis endorses EHT.” (Molly McConnell Dow, February 27, 2017);
“Have you learnt somebody with Parkinson’s? Nerium EHT CAN HELP!” (Joanie Sullivan, June 15, 2016);
“Michael J Fox endorses EHT!” (Alex Forero, October 27, 2015);
“Do you or anybody you recognize endure from Parkinson’s Illness? In a examine printed in 2013, EHT, the principle ingredient in Nerium’s EHT complement was examined and located to alleviate the signs of Parkinson’s.” (Skincaring.Nerium.com, December 17, 2015);
“Love my Princeton scientists. Take heed to this video explaining EHT advantages…geared towards Parkinson’s. Alzheimer’s. Dementia.” (Amy E. Tate-Matthus, March 10, 2016).
“EHT for mind well being . . . #alzheimers #dementia . . . #parkinsonsdisease #parkinsons.” (Carrie Skowronek Instagram, Sept. 1, 2019).
“Analysis and Funded by the Michael J. Fox Basis . . . Had a concussion . . . household historical past of Alzheimer’s or Parkinson’s . . . Mind well being is so necessary!!! #EHT.” (Maria Cipriano Instagram, October 10, 2019).
There are not any human scientific trials displaying that EHT prevents, reduces the chance of, or treats Parkinson’s illness, and there are not any research in any way, together with human scientific trials, which have been performed on EHT Merchandise themselves associated to that declare.
The FTC collected proof of false medical claims regardless and sued Signum too.
The FTC accuses Neora, Jeff Olson and Signum of a number of FTC Act violations, together with;
- working an unlawful pyramid scheme (Nerium Worldwide and Jeff Olson);
- making false incomes claims (Nerium Worldwide and Jeff Olson);
- making false or unsubstantiated efficacy claims (all defendants);
- making false institution claims (all defendants);
- offering Neora distributors with the means and instrumentalities for the fee of misleading acts and practices (all defendants)
The FTC alleges client harm because of Neora, Olson’s and Signum’s actions, and has requested for an injunction, disgorgement of ill-gotten features and authorized prices.
In line with the November 1st press-release issued by the FTC, Signum has opted to settle the lawsuit.
On the time of publication the FTC’s lawsuit is but to indicate up on Pacer. When it does, we’ll proceed to trace the case and supply you updates.
Replace 2nd November 2019 – Neora has filed its personal November 1st lawsuit towards the FTC.
Replace eleventh September 2020 – Neora’s lawsuit towards the FTC has been dismissed.
Replace eleventh October 2020 – The FTC’s case has been given a November 2022 trial date.