Awakend co-founder Danelle Meoli continues to mislead distributors and the public on “exclusive rights”, pertaining to the yet-to-be-shipped supplement Zenith.
BehindMLM has been tracking the Zenith patent controversy since August.
The dispute, between TriPharma (now dba Vietal Nutrition) and Family First Business Ministry (FFBM), is scheduled for trial on March 28th, 2023.
Pending the outcome of the ongoing California lawsuit, the court noted the patent dispute remains “unsettled”.
That suit remains ongoing. The rights to the Patent remain unsettled.
Despite this, Meoli is telling Awakend distributors the company has “exclusive rights” to the disputed patent.
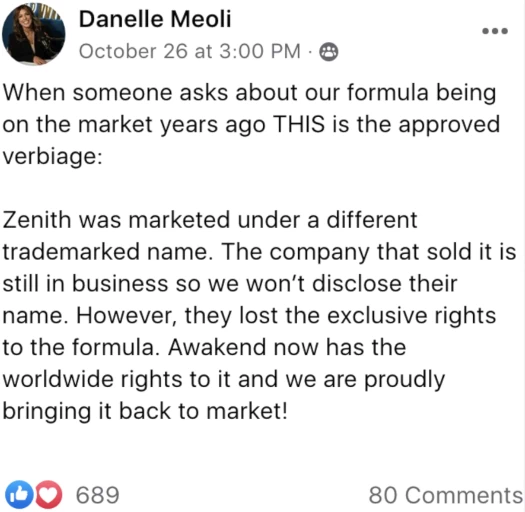
When someone asks about our formula being on the market years ago THIS is the approved verbiage:
Zenith was marketed under a different trademarked name.
The company that sold it is still in business so we won’t disclose their name.
However, they lost the exclusive rights to the formula.
Awakened now has the worldwide rights to it and we are proudly bringing it back to market!
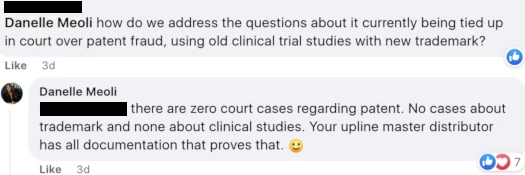
When asked by an Awakend distributor about the court case referenced above, Meoli flat-out lied:
The disputed patent issue being unsettled isn’t my interpretation or opinion, I’ve provided that verbatim from a court order.
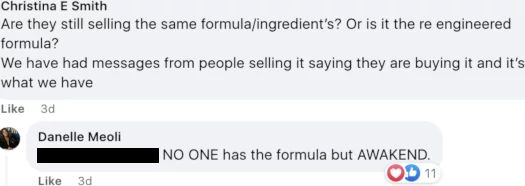
In the same private Awakend FaceBook group, another distributor pushed back on exclusivity;
Meoli’s answer is again believed to be false. FFBM’s and Vietal Nutrition’s formulas are based on the same patent and ingredients (that’s why this is a legal issue).
Getting back to the patent dispute lawsuit, (TriPharma v. FFBM) et. al.), on October 20th BehindMLM reported that FFBM’s counterclaims against Awakend had been dismissed.
Four counterclaims were dismissed due to “newly discovered facts” being absent from the original filing. FFBM’s other two counterclaims were dismissed for being
so vague and conclusory that they fail to give rise to plausible claims against Tripharma.
The court granted FFBM permission to refile an amended counterclaim, which it did on October 26th.
In March 2011 FFBM paid millions of dollars to purchase all rights to the ’892 Patent, all rights to Trisynex, all rights to the UConn study and other clinical studies conducted on Trisynex, and all rights to the EJAP Publication.
FFBM has possessed the exclusive rights to the Trisynex product, the clinical studies, and the exclusive right to use and refer to the EJAP Publication (collectively referred to herein as “the Intellectual Property”) since October 1, 2014.
FFBM, however, has recently discovered that earlier this year the Tripharma Defendants falsely represented to the multi-level marketing company Awakend and its representatives that Tripharma had the exclusive rights to the Intellectual Property and that Tripharma could “assign” these rights to Awakend.
Through this fraudulent licensing arrangement, Awakend and the Awakend Individual Defendants are now falsely claiming it has the exclusive rights to the ’892 Patent (a patent that has EXPIRED) and the exclusive rights to the Intellectual Property, including the UConn Study and resulting EJAP Publication.
Awakend and the Awakend Individual Defendants have purported to “rebrand” the Trisynex product as Awakend’s “own product,” called Zenith.
As part of this “rebranding,” the Counter-Defendants have intentionally doctored the EJAP Publication to replace the tradename Trisynex (which was the actual subject of the UConn study) with “Zenith.”
BehindMLM covered Awakend’s doctored Zenith study in August. It has since been pulled from circulation.
FFBM brings this Counter-Complaint to pursue damages arising from Counter-Defendants’ fraudulent scheme, as well as injunctive relief to prevent Awakend from continuing to market, sell, or distribute its “Zenith” product and to stop Counter-Defendants from using or referring to the Intellectual Property and/or the ’092 Patent.
At this stage I don’t know if FFBM’s counterclaim is going to have an impact on the scheduled March 2023 jury trial. I’ll continue to monitor the case docket for updates.
Footnote: BehindMLM calling out Meoli’s misinformation shouldn’t be interpreted as “going in to bat” for FFBM.
BehindMLM has no stake in the outcome of this dispute, I’m just reporting on developments pending the final outcome of the case. We’ll cover the outcome regardless of whether TriPharma or FFBM prevails.
I maintain that Awakend should not have begun taking fees from consumers when the patent behind their flagship supplement (which for some reason still hasn’t shipped yet), remains in dispute.
We’d also like to Meoli stop plying Awakend distributors with misinformation.